- Home
- »
- Help Desk
- »
- Repository Specific FAQs
Repository Specific FAQs
Q1. Who do I contact for help with the cdRNS system?
Please contact NINR Operations at NINR-BRICS-OPS@mail.nih.gov
Q2. Does collecting information to create GUIDs using the Global Unique Identifier (GUID Tool) increase the likelihood of breach of confidentiality?
Answer: No, the GUID Tool is a customized software application that generates a Global Unique Identifier for each study participant. The GUID is made up of random alpha-numeric characters and is NOT generated from PII/PHI. The GUID allows researchers to share data specific to a study participant without exposing personally identifiable information (PII). As such, it has been approved by the NIH Office of General Counsel. GUID Generation complies with HIPPA regulations for the protection of PII/PHI.
The GUID process has two important attributes:
- PII is never sent to the cdRNS® system
- The GUID is a random number not generated from PII/PHI
Q3. If we are collecting data from Hispanic patients, do we need to submit these data?
Answer: Currently we do not have forms in Spanish in the cdRNS system.
Q4. We have data collected against forms that are not in cdRNS – can we submit these data? And is there a plan to add these forms?
Answer: For now, only the forms you see in the cdRNS are approved, and therefore only data against these forms should be submitted. Approved forms can be found here: https://cdrns.nih.gov/data/data-dictionary#extramural-form-pdfs
Q5. How do we generate GUIDS, if we do not have all the required fields? Some patients will not answer these questions.
Answer: The cdRNS requires GUID’s to be generated using a specific set of questions. The required fields to generate a GUID can be found here: https://cdrns.nih.gov/how-to/guid. This information will be helpful when submitting your study for IRB approval. A pseudo GUID is possible in certain situations. Please contact the BRICS OPS Team for this approval.
Q6. For city of birth, some patients do not have or do not provide this, or we have not collected this data. Can we use “unknown” instead?
Answer: While using “unknown” instead of city of birth works to generate a GUID, we do not recommend using this option. The main reason for this is that if 2 people have the same information for everything else except city of birth, validation will not pick that up and there is no way to distinguish these 2 individuals and their data. A pseudo GUID is possible in certain situations. Please contact the BRICS OPS Team for this approval.
Q7. Can we submit data at specific times in the month instead of a live capture?
Answer: There is no restriction on submitting data either as an active capture or as entries to the ProFoRMS module at fixed timepoints. The best way to enter data is as it is being collected so that data entry is not backed up.
Q8. Of the forms in the cdRNS, which forms are required to be completed at baseline?
Answer: There are 2 required forms, Demographics and Diagnosis, that MUST be completed at the first visit/baseline visit. Though optional, the Social Determinants of Health form is recommended, to be completed at baseline, since it captures additional demographics information. Approved forms can be found below and are found here: https://cdrns.nih.gov/data/data-dictionary#extramural-form-pdfs.
Check with your program officer for other study required forms prior to data collection.
Q9. Will the data be stored permanently in cdRNS data repository? Or is there a specific time duration associated with storing the data in cdRNS?
Answer: Data are expected to be stored as designated by NINR. Please refer to the cdRNS data policy found here: https://www.ninr.nih.gov/aboutninr/policies.
Q10. How will the data in the cdRNS repository be used?
Answer: Data with the public/shared status in the cdRNS repository can be queried by users with permissions through the Query Module beginning one year after your study ends. See data sharing policy found here: https://www.ninr.nih.gov/aboutninr/policies. You can also check with your NINR program officer.
Q11. What is the recommendation to lock and submit the data when using the ProFoRMS module?
Answer: Once data have been collected via the ProFoRMS module, the recommendation is for the data managers (or assigned personnel) to check the data for accuracy and then lock and submit the data.
Q12. Is cdRNS a PC only system?
Answer: cdRNS is a web based system that can be accessed on any browser. Safari allows the users to change the “user agent” to allow browsing in other internet browsers, such as Internet Explorer. Please see instructions below:
- Go to safari > preferences > advanced > click on “show develop menu” in menu bar
- In “develop menu” on menu bar > “user agent” > “internet explorer 11”
- Access cdrns.nih.gov
Q13. If I stop during data entry and return later, how do I find where I stopped?
Answer:
Please see instructions and snapshots below:
In ProFoRMS module:
- Go to “Collect data” (snapshot 1)
- Click on “My collections” (this should give you a snapshot of forms that have started data collection (snapshot 2)
- Select your subject
- Click on “Edit”
If you need further assistance please contact NINR operations, NINR-BRICS-OPS@mail.nih.gov
Snapshot 1:
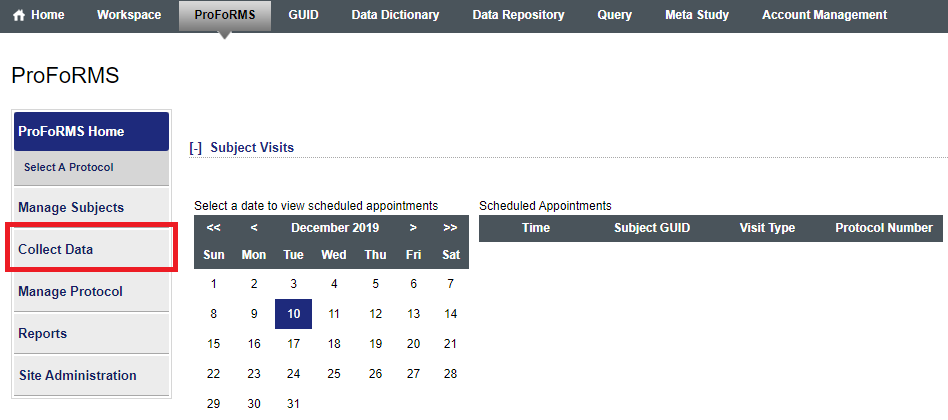
Snapshot 2:
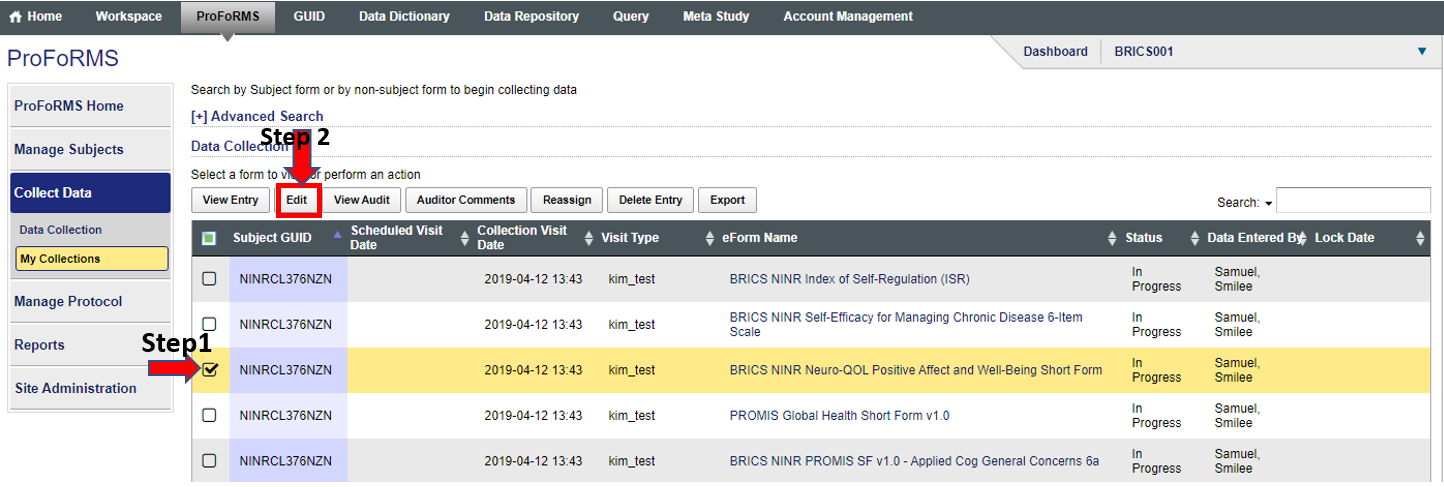
Snapshot 3:
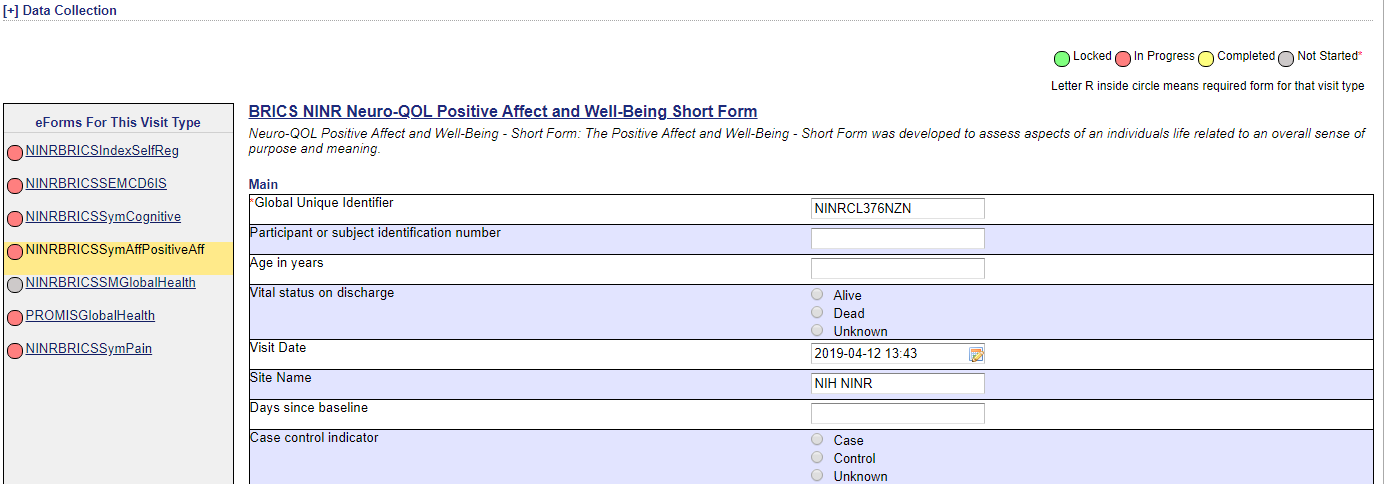
Q14. If I have locked my data, and want to return and add a comment on a participant, is there a way to do this?
Answer: Please see instructions and snapshots below:
In ProFoRMS module
- Go to “Collect data”
- Click on “My collections”
- Select your subject with the locked form (snapshot 1)
- Click on “Edit”
- Under the Main Group (snapshot 2)
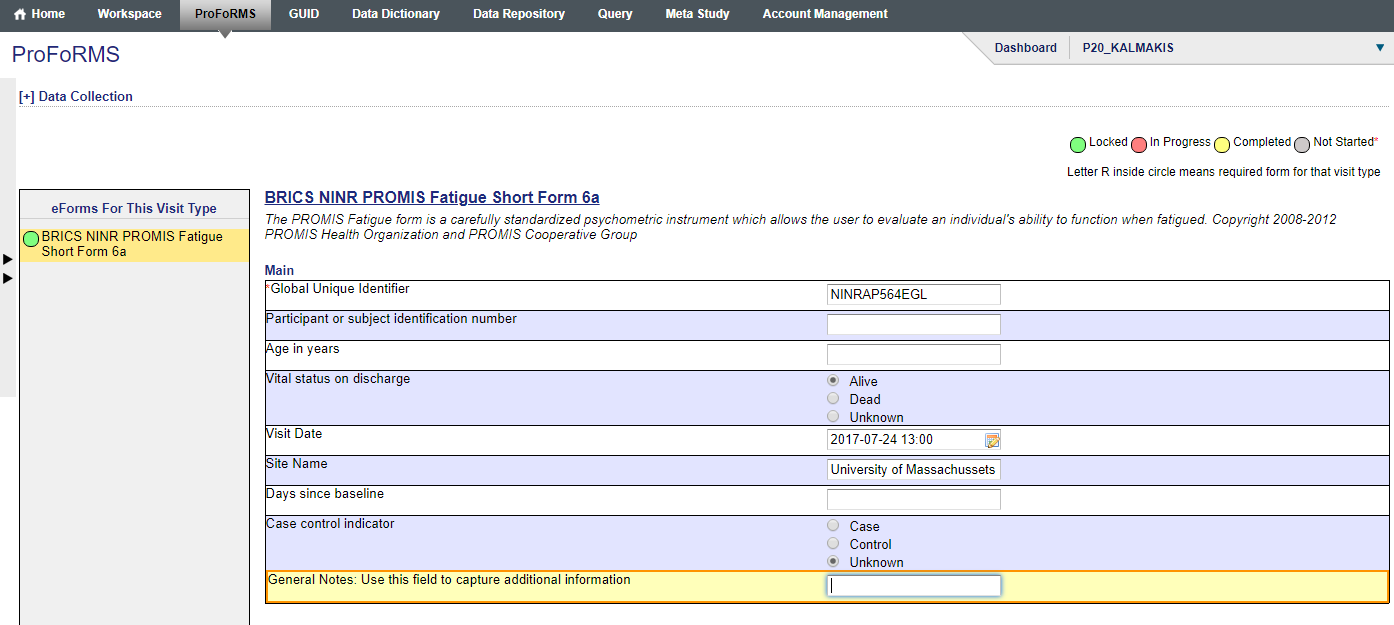
- Go to “General Notes: Use this field to capture additional information” and enter comments
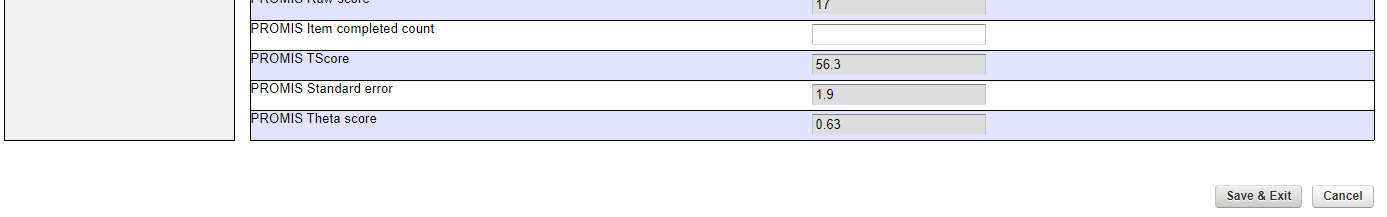
- Proceed to “Save and Exit” (snapshot 3)
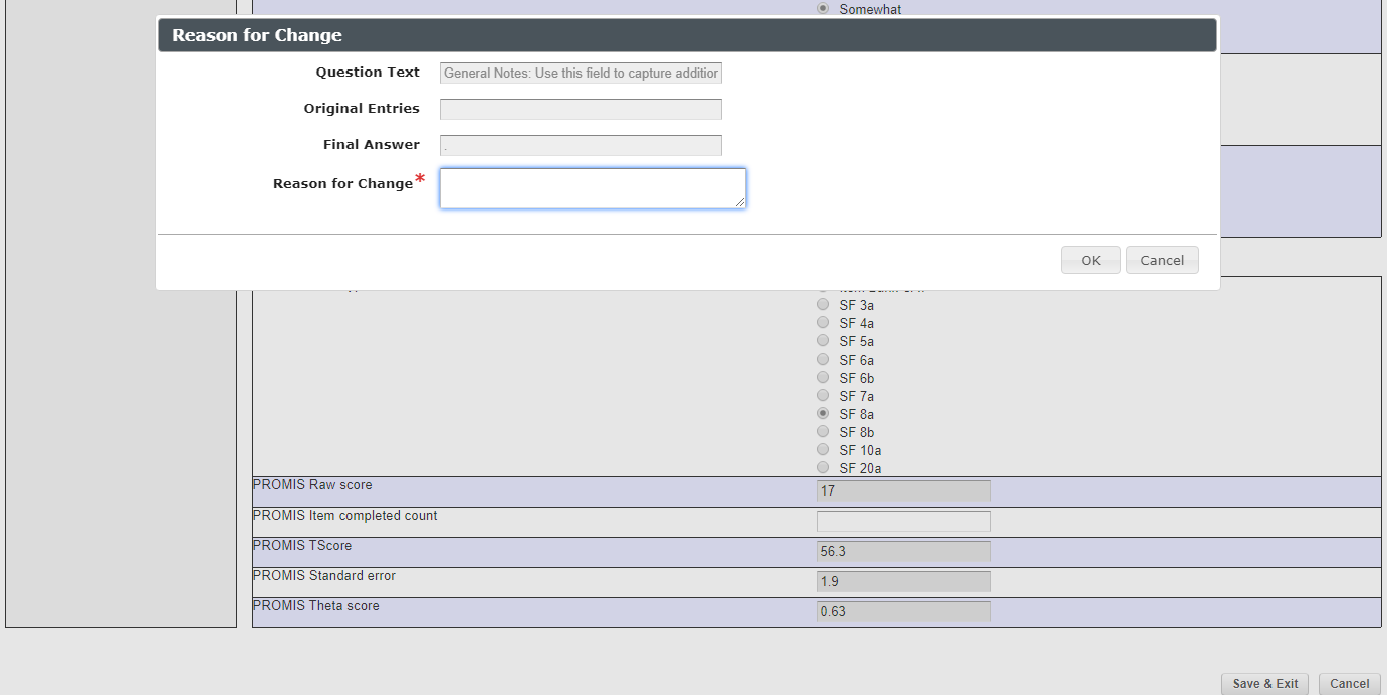
- A box with “Reason for change” appears (snapshot 4)
- Enter the reason.
- Click “Ok”
- Now click on “Save and Exit” again.
If you need further assistance please contact NINR operations, NINR-BRICS-OPS@mail.nih.gov
Snapshot 1:
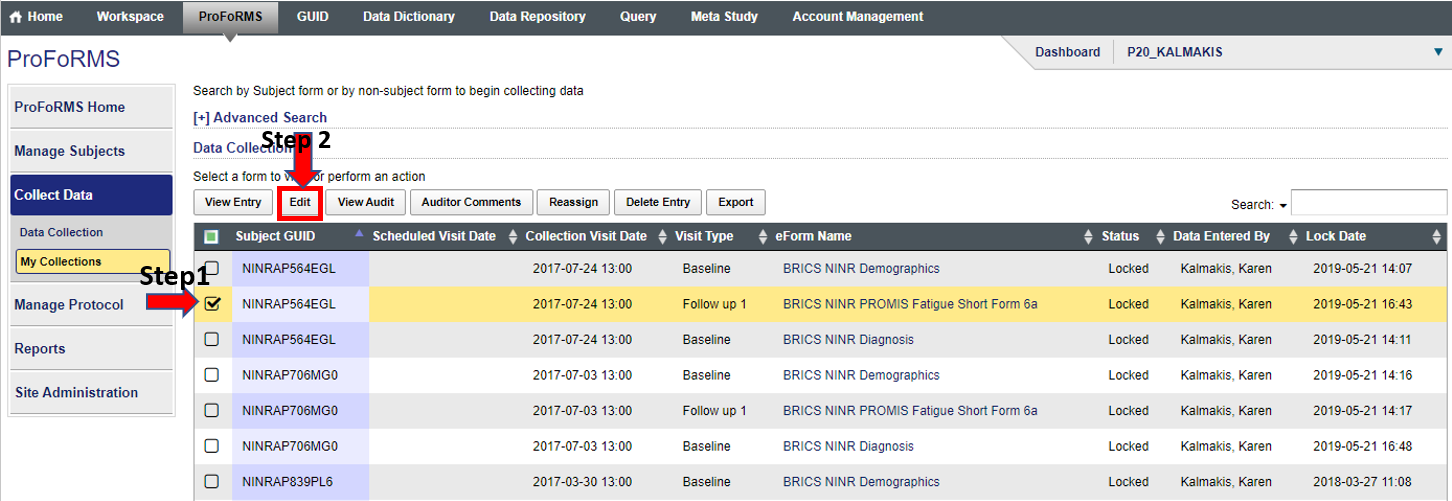
Snapshot 2:
Snapshot 3:
Snapshot 4:
Q15. How do I easily go from one form to the next form during data collection?
Answer: Please see instructions and snapshots below;
- In ProFoRMS module:
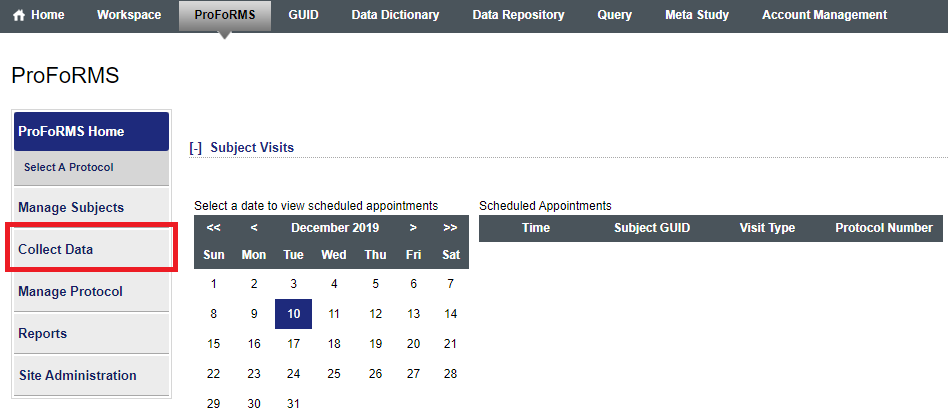
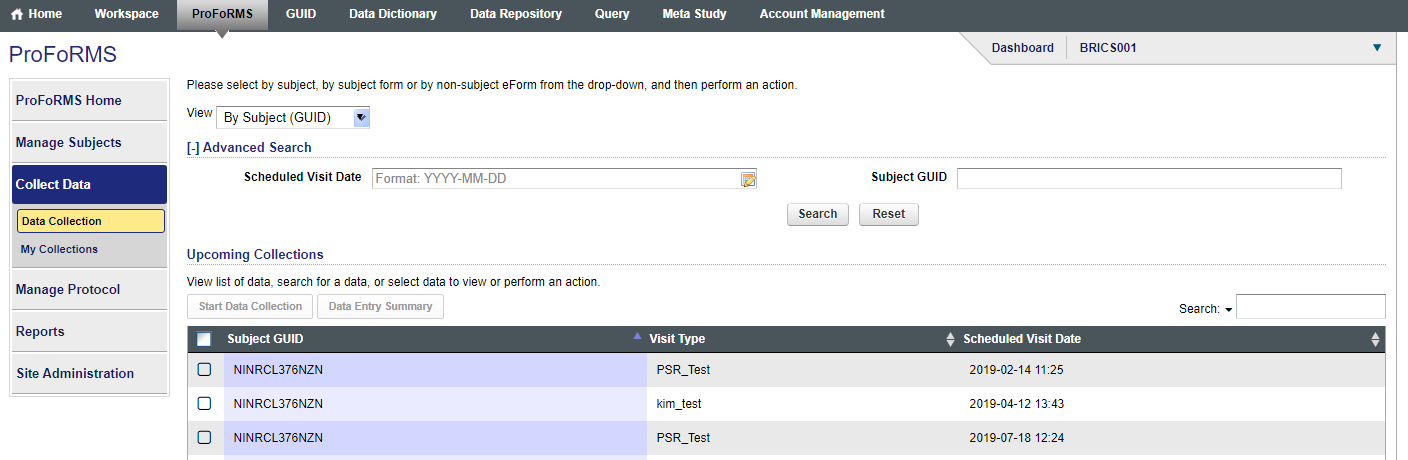
- Go to “Collect data” (snapshot 1)
- Select your subject
- Click on “Start data Collection” (snapshot 2)
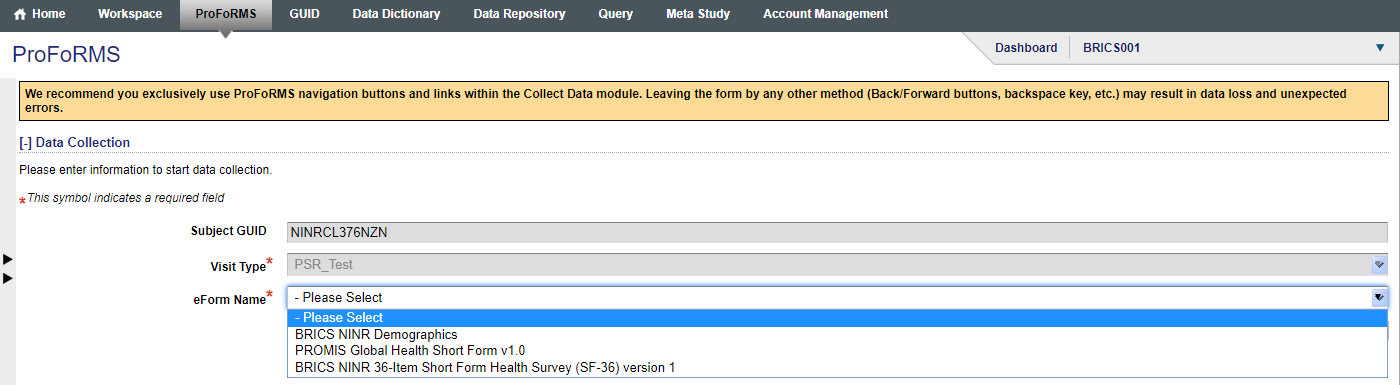
- Select your form from the drop down (snapshot 3)
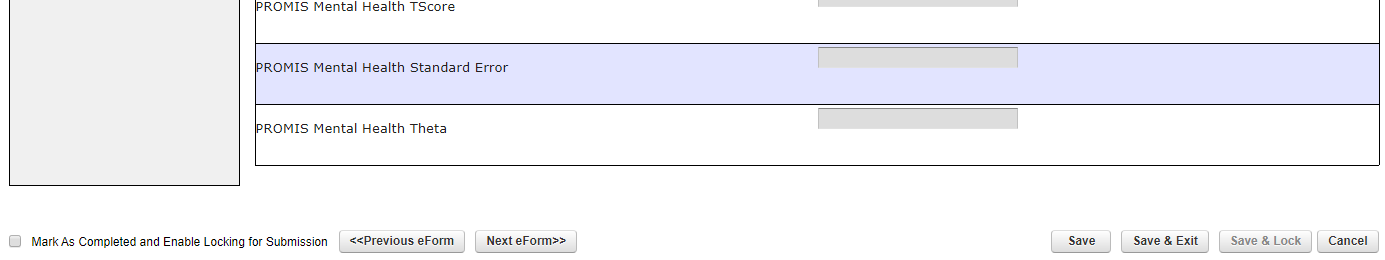
- After entering data, at the bottom of the screen select -> Click on “Next eForm” (snapshot 4)
Snapshot 1:
Snapshot 2:
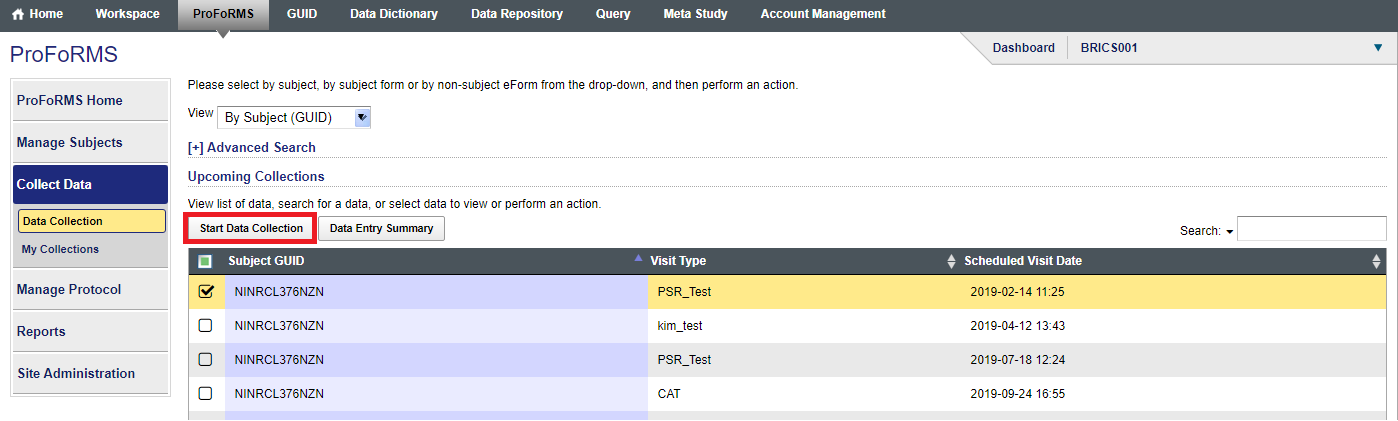
Snapshot 3:
Snapshot 4:
Q16. How can I search for a subject using the subject ID instead of the GUID before I add data?
Answer: Currently the search feature for subjects is set to the default subject label of GUIDS (snapshot 1). Please send a request to NINR operations NINR-BRICS-OPS@mail.nih.gov, if you would like this to be changed and they will change the default subject label from GUID to Subject ID for your study(snapshot 2). Snapshot for subject labels included below.
Snapshot 1: Subject Label: GUID
Snapshot 2: Subject Label: Subject ID
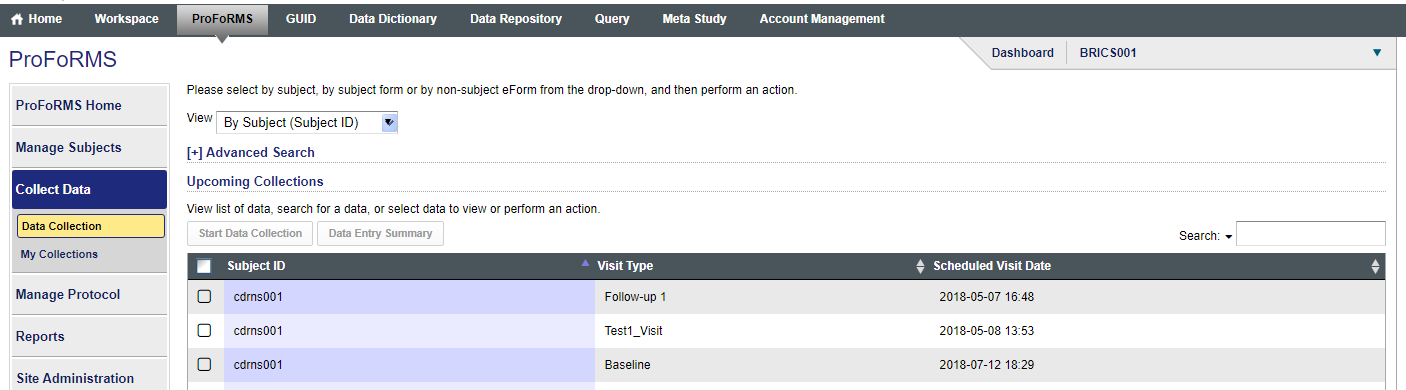
Q17 What is the guidance for entering data if the visit type is missed by a subject?
Answer: The recommendation is to enter data to the accurate visit type. For example: If the study has 3 visit types: visit 1, visit 2 and visit 3 and when subject misses visit 2, data collected in visit 3 should be entered in visit 3 and not in visit 2.
Q18. I will be a data manager/project manager to collect and enter data. Do I need a user account or can we share an account?
Answer: Each individual entering data must have their own account, shared accounts are not allowed. All cdRNS users MUST attend the required training prior to any data collection and data entry. We also recommend that Center PIs have an account to insure that data is entered and locked regularly. The Center PI has the ultimate responsibility to ensure that the required data is entered and locked according to grant expectations.
Q19. What kind of language should be included in the consent form for data sharing and storage and deidentifying data for the cdRNS?
Answer: NINR has the expectation that investigators will use broad consent language that allows for the storing, sharing, and use of human subjects data for future research, even if such future research may be unrelated to the original study or disorder for which the data were collected.
NINR expects investigators to consider including such language (or similar language as mandated by your IRB) in your consent forms in order to ensure that data are available for storing, sharing and use in the future. This language should be included in the draft consent submitted with your application submitted to NINR in preparation for your application if funded. Biomarker data should be submitted according to the policy at https://cdrns.nih.gov/policies/intramural/data-sharing-policy.
For further questions on language for consent, please contact NINR Program staff.
